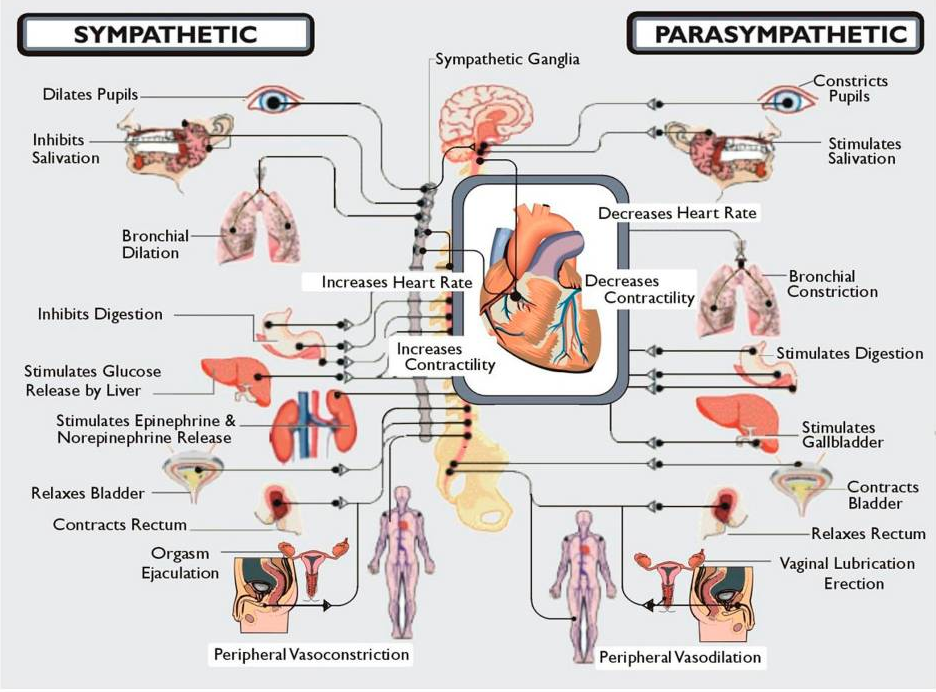
Well, basically POTS is like this. When a person stands up their blood flows to their heart and brain and then down to their waist and feet. In someone with POTS when the person stands the blood flows to the feet first and then by the time the body knows this the heart has started working really hard to get the blood to flow up to the heart and bairn. By that time the POTS patient is dizzy and out of breath and sweating. This is the one disorder that makes my life so very very hard. I can deal with the pain of Ehlers Danlos Syndrome and R.A. and Lupus and the many other disorders that cause pain but the POTS is so life altering for me that it has not only destroyed my dreams but it has stolen my dignity from me as I am just unable to take care of myself.
I would like to add to this extreme sweating, being out of breath, cooling down to the point of my skin becoming white and clammy and then freezing which no amount of blankets or heat will warm you up, and extreme fatigue. I'm sure there are other symptoms that happen but these mentioned in the picture and what I added happen every single time I take a shower!!! It is just awful!
I am going to provide the links of different blogs that will explain POTS and the way it can control the body and an article about a study of patients with POTS and how it is proven that there is a lower blood volume in these people. It is important to know this because the outcome of blood test never show what is wrong with the POTS patient because the wrong values are been used since most POTS patients don't have the same amount of blood as non POTS people. For instance, (This is not an exact way to do this, plus it involves math and math and I are by no means friends so please take this as only a what if.) if you have two bottles of water, one being 100% full and one being 80% full to get the same outcome of a test of the two from the 100% full bottle say 4 TBSP would need to be drawn and from the 80% full bottle say 5 TBSP would need to be drawn to get the same results of the outcome. If this is not done then the results of the test for the 80% full bottle would be about 20% higher than the actual result causing the POTS patients needs to be overlooked causing the patient to remain weak, tired and sick not being allowed to get the necessary medication, saline IV, vitamins and any other products that would help the POTS patient have a more productive life. Another way this could be done is to take the outcome of the POTS patients blood test and multiply it by .8 or to adjust the scale or range of normal blood levels for a test you would take both sides of the scale and multiply by 1.25. to give you a better idea of where the POTS patient falls in the blood test ranges. Again, this is not an exact way to do this because the POTS patient, unless they are tested to see how much blood volume they have, will not have the correct numbers to multiply with. It cannot be assumed that all POTS patient have the same lower level of blood volume, nor does every POTS patient have a lower level of blood volume than non POTS people.
So how much blood is in the human body? http://wonderopolis.org/wonder/how-much-blood-is-in-your-body/
Blood is composed of cells and plasma. The cells include red blood cells, white blood cells, and platelets.So how much blood is in the human body? http://wonderopolis.org/wonder/how-much-blood-is-in-your-body/
The red blood cells carry oxygen from the lungs and give blood its red color. The white blood cells fight infections. The platelets help form clots to stop bleeding in case of a cut.
All these cells float in the liquid plasma, which is mostly water. Plasma also contains nutrients, electrolytes, hormones, and protein antibodies to fight infection.
The amount of blood in a human body varies, depending on factors such as age, sex, overall health and even where a person lives. For example, men tend to have more blood than women of comparable size and weight.
Interestingly, people who live at high altitudes may have up to two liters of extra blood compared to those who live at lower altitudes. Because the air at higher altitudes has less oxygen, people who live at high altitudes need extra blood to deliver the right amount of oxygen to their lungs.
Scientists estimate the volume of blood in a human body to be approximately 7 percent of body weight. An average adult body with a weight of 150 to 180 pounds will contain approximately 4.7 to 5.5 liters (1.2 to 1.5 gallons) of blood. An average child with a body weight of 80 pounds will have approximately half the amount of blood as an adult.
Blood carries out many critical functions in the body. It transports nutrients and oxygen to the body’s cells. It also takes away waste from those cells. Blood also moves hormones and chemicals around the body.
You might be surprised to learn that blood also plays a special role in regulating body temperature. When your body heats up, blood helps keep the temperature steady by transferring extra heat to the skin, where it can be released from the body.
As part of the immune system, blood also helps fight disease. If you scrape your knee at the park, the platelets in the blood begin to clot to help stop the bleeding. This self-repair function prevents further blood loss, which could be fatal in cases of massive bleeding.
This link I am providing is a link to explain what POTS is.
http://edstoday.org/eds-awareness/associated-disorders
Postural Orthostatic Tachycardia Syndrome (POTS)
“One of the most debilitating complications of Ehler-Danlos Syndrome EDS is a type of dysautonomia called Postural Orthostatic Tachycardia Syndrome or POTS. Dysautonomia is the result of our autonomic nervous system ceasing to function properly. Our autonomic nervous system regulates functions that our body does automatically such as digestion, breathing, heart rate, blood pressure, body temperature, blood sugar regulation, hormonal/endocrine imbalances, and our sleep cycle.”
~Dr. Diana Driscoll~
This link is to a blog that explains a little about low blood volumes in POTS patients and what life is like with this condition. http://potsgrrl.blogspot.com/p/in-support-of-iv-saline-therapy-for.html
Lastly, I am adding an article about the testing of POTS patients that explain the testing done to determine that POTS patients have a lower blood volume than people without POTS. I hope that you are able to understand more about this condition and to learn enough about it to know that it doesn't strike only patients with Ehlers Danlos Syndrome and although many people that get POTS can be cured of it, in Ehlers Danlos Syndrome there is no cure or fix for it. It remains a very disabling disorder that is hard to explain to friends and family or to get them to believe that the POTS patients can be extremely ill as a result of it.
To see the References please visit the article at
http://circ.ahajournals.org/content/111/13/1574.full
Arrhythmia/Electrophysiology
Renin-Aldosterone Paradox and Perturbed Blood Volume Regulation Underlying Postural Tachycardia Syndrome
Satish R. Raj, MD;
Italo Biaggioni, MD;
Paula C. Yamhure, RN;
Bonnie K. Black, RN, NP;
Sachin Y. Paranjape, BS;
Daniel W. Byrne, MS;
David Robertson, MD
+Author Affiliations
From the Divisions of Clinical Pharmacology (S.R.R., I.B., D.R.) and Cardiovascular Medicine (P.C.Y., B.K.B., S.Y.P.), Departments of Medicine, Pharmacology (S.R.R., I.B., D.R.), Biostatistics (D.W.B.), and Neurology (D.R.), Vanderbilt University, Nashville, Tenn.
Reprint requests to Satish R. Raj, MD, AA3228 Medical Center North, Vanderbilt University, 1161 21st Ave S, Nashville, TN 37232-2195. E-mail satish.raj@vanderbilt.edu
Next Section
Abstract
Background— Patients with postural tachycardia syndrome (POTS) experience considerable disability, but in most, the pathophysiology remains obscure. Plasma volume disturbances have been implicated in some patients. We prospectively tested the hypothesis that patients with POTS are hypovolemic compared with healthy controls and explored the role of plasma renin activity and aldosterone in the regulation of plasma volume.
Methods and Results— Patients with POTS (n=15) and healthy controls (n=14) underwent investigation. Heart rate (HR), blood pressure (BP), plasma renin activity, and aldosterone were measured with patients both supine and upright. Blood volumes were measured with 131I-labeled albumin and hematocrit. Patients with POTS had a higher orthostatic increase in HR than controls (51±18 versus 16±10 bpm, P<0.001). Patients with POTS had a greater deficit in plasma volume (334±187 versus 10±250 mL,P<0.001), red blood cell volume (356±128 versus 218±140 mL,P=0.010), and total blood volume (689±270 versus 228±353 mL,P<0.001) than controls. Despite the lower plasma volume in patients with POTS, there was not a compensatory increase in plasma renin activity (0.79±0.58 versus 0.79±0.74 ng · mL−1 · h−1, P=0.996). There was a paradoxically low level of aldosterone in the patients with POTS (190±140 pmol/L versus 380±230 pmol/L;P=0.017).
Conclusions— Patients with POTS have paradoxically unchanged plasma renin activity and low aldosterone given their marked reduction in plasma volume. These patients also have a significant red blood cell volume deficit, which is regulated by the renal hormone erythropoietin. These abnormalities suggest that the kidney may play a key role in the pathophysiology of POTS.Key Words:
tachycardia
renin
nervous system, autonomic
blood volume aldosterone
Received September 26, 2004; revision received December 14, 2004; accepted December 21, 2004.
Postural tachycardia syndrome (POTS) is the most common disorder among patients seen at several centers specializing in diseases of the autonomic nervous system. It affects an estimated 500 000 people in the United States alone.1 POTS (excessive increase in heart rate [>30 bpm] on standing, associated with orthostatic symptoms in the absence of orthostatic hypotension) can produce substantial disability among otherwise healthy people.
The pathophysiology of POTS is poorly understood. Many of these patients have elevated levels of plasma norepinephrine, particularly when upright.2 Subgroups of patients have a primary hyperadrenergic state,3 whereas others have partial dysautonomia that affects the lower limbs.4–7 The role of blood volume in the pathogenesis of POTS is unclear. Some investigators have found that patients with POTS have deficits in their plasma volumes8,9and that some patients improve after acute10 or chronic9 plasma volume expansion. Conversely, other investigative groups have reported normal plasma volumes in patients with POTS.11,12
The renin-angiotensin-aldosterone system plays a key role in the neurohormonal regulation of plasma volume in humans.13 In response to hypovolemia, plasma renin activity and angiotensin II would be expected to increase to promote blood volume expansion. Angiotensin II promotes sodium and water retention, both directly by stimulating sodium reabsorption in the proximal tubules14 and indirectly by stimulating aldosterone secretion.15The mineralocorticoid aldosterone governs sodium transport at several sites in the kidney. Jacob et al8 have reported that some patients with POTS have low plasma renin activity despite a low plasma volume, which suggests that a perturbation in the renin-angiotensin-aldosterone axis might have a role in the pathophysiology of POTS.
In this prospective, controlled study, we tested the hypothesis that patients with POTS have a deficit in plasma volume. We also explored the regulation of plasma volume by renin and aldosterone.
Previous SectionNext Section
Methods
Subjects
Patients referred to the Vanderbilt University Autonomic Dysfunction Center with POTS between October 2002 and November 2003 were candidates for inclusion in this study. Patients met the current criteria for POTS.16 Briefly, patients developed symptoms of orthostatic intolerance accompanied by a heart rate rise ≥30 bpm (or a rate that exceeds 120 bpm) that occurred within the first 10 minutes of standing or head-up tilt, without any evidence of orthostatic hypotension (a fall in blood pressure of >20/10 mm Hg). Patients had at least a 6-month history of symptoms, in the absence of another chronic debilitating disorder or prolonged bed rest, and were at least 18 years of age. Healthy control subjects (who did not meet criteria for POTS, and were at least 18 years of age) underwent all of the same protocol elements. Patients and controls were free of medications that could impair autonomic tone17 and were not taking fludrocortisone for at least 5 days before testing. The Vanderbilt University Investigational Review Board approved this study and written informed consent was obtained from each subject before the study began.
Protocol
Study investigations were performed at the Elliot V. Newman Clinical Research Center at Vanderbilt University. For 3 days before testing, subjects consumed a diet that contained 150 mEq of sodium per day and 70 mEq of potassium per day. The diet was free of caffeine-containing beverages.
Supine and Upright Posture Study
Heart rate, blood pressure, aldosterone, plasma renin activity, and plasma norepinephrine and epinephrine were assessed after overnight rest with subjects in the supine position and again after subjects had been standing for up to 30 minutes (as tolerated). The standing test was performed to assess the hemodynamic and biochemical responses to increased central hypovolemia (accentuated by the gravitational stress). For catecholamine measurements, blood was collected in plastic syringes and immediately transferred to chilled vacuum tubes with EGTA and reduced glutathione (Amersham International PLC) and immediately put on ice. The plasma was separated by refrigerated centrifugation at −4°C and stored at −70°C until the assay. Concentrations of norepinephrine and epinephrine were measured by batch alumina extraction, followed by high-performance liquid chromatography for separation with electrochemical detection and quantification.8 Plasma renin enzymatic activity was assayed by conversion of angiotensinogen to angiotensin I by a radioimmunoassay technique (antibodies from IgG Corporation)18and reported in nanograms of angiotensin I per milliliter per hour. Blood for aldosterone was collected in chilled vacuum tubes without preservative, and the serum was extracted and sent to the laboratory on ice. Serum aldosterone was measured by radioimmunoassay (DPC Coat-a-Count, Diagnostic Products Corp). The aldosterone-to-plasma renin activity ratio was calculated with the conventional units for aldosterone (ng/dL; 1 ng/dL=27.7 pmol/L) and plasma renin activity (ng · mL−1 · h−1) and reported without units.
Blood Volume Assessment
Plasma volume was determined by the indicator dye-dilution technique. In the morning after an overnight fast, patients were placed in a supine position for a minimum of 60 minutes before collection of the baseline sample. A 20-gauge intravenous catheter was placed in an antecubital vein, and blood samples could be obtained without stasis. A baseline venous sample of 5 mL was collected before injection of the tracer. With a prefilled 1-mL syringe, up to 25 μCi of 131I-labeled human serum albumin (Volumex, Iso-Tex Diagnostics Inc) was injected into the antecubital vein and flushed with 30 mL of normal saline. Starting at 12 minutes after injection, 5 mL of venous blood was collected at 6-minute intervals until 30 minutes after injection (5 samples, including baseline sample). Hematocrit was measured in duplicate from each sample after 10-minute centrifugation at 11 500 rpm on an International Equipment Co microcapillary centrifuge and read on an International Equipment Co microcapillary tube reader. Plasma radioactivity was measured in duplicate and averaged (for each sample and a reference standard) with an automated counter (BVA-100 Blood Volume Analyzer, DAXOR Corporation). A least-squares regression of the volume of distribution at each time point was automatically performed to determine the volume of distribution at the time of injection. Plasma volume was determined as the volume of distribution of albumin.
Total blood volume was calculated from measured plasma volume and microcapillary venous antecubital hematocrit corrected for the plasma-packing ratio (0.99),19 the ratio of mean body hematocrit to peripheral (measured) hematocrit (0.91),20 and the effects of heparin within the sampling syringe (0.97).21
Red blood cell volume was calculated as the difference between total blood volume and plasma volume. This DAXOR method of red blood cell volume assessment was recently found to correlate well with the traditional 51Cr red blood cell-labeling method (Pearson correlation R=0.96), with a mean difference between the techniques of 0.9% (personal communication with Dr. Howard Dworkin, William Beaumont Hospital, Royal Oak, Mich).
Ideal plasma and total blood volume was determined on the basis of the height, weight, and gender of the individual subject.22Individual “deficits” in plasma volume, red blood cell volume, and total blood volume were calculated as the ideal minus measured volume (in milliliters), or this difference divided by the ideal volume (percentage).
Plasma Volume Shift With Upright Tilt
All studies occurred between 10 AM and noon in a quiet, dimly lit room at a comfortable ambient temperature (21°C to 24°C). An antecubital venous catheter was inserted (if not already in situ and functioning) for blood sampling at least 15 minutes before the beginning of the test, with the patient supine. Subjects were tilted head-up to 60° for 30 minutes or until the subject experienced presyncope that required test termination.
Blood was drawn at baseline and then at 5, 10, 15, 20, and 30 minutes of tilt for measurement of hematocrit in quadruplicate (see Blood Volume Assessment section for details of hematocrit assessment). Relative changes in hematocrit from baseline were used to calculate the change in plasma volume with upright tilt. The percentage change in plasma volume (ΔPV%)=100×[(HctBaseline−HctTime)/HctTime]× [1/(1−HctBaseline)], with the absolute change in plasma volume (ΔPV)=ΔPV%×measured plasma volume (where HctBaseline is hematocrit before tilt, and HctTime is hematocrit at a given time after tilt).23 ΔPVEnd and ΔPV%End were defined as the ΔPV at the time of the last measured hematocrit before tilt termination. ΔPVEnd was used in place of the individual late time points to minimize the confounding effect of late data dropout due to premature tilt termination. A negative value reflects a shift in plasma volume out of the vascular space.
Gender Analysis
POTS is a disorder that affects women more often than men. In addition to an overall analysis that included all subjects, a separate analysis was performed that included only female subjects. This was to ensure that the results were not skewed by the small number of men.
Statistical Analysis and Sample Size Calculations
Our primary endpoint was the plasma volume deficit (measured plasma volume minus ideal plasma volume). The null hypothesis was that the plasma volume deficit would not be statistically different between patients with POTS and control subjects. We calculated the size of our required sample after determining that a 7.5% deficit in plasma volume in patients with POTS would be clinically significant. We did not expect the control subjects to have a plasma volume deficit. Assuming a pooled SD of 5% (giving an effect size of 1.5), a sample size of 13 subjects in each group would give 95% power to detect a statistically significant difference with a Student t test with a 2-sided significance level of 0.05.24Differences between groups were analyzed with the Student t test. The Mann-Whitney U test was also used to confirm the results obtained from the Student t test, and the significance of the reported parameters was not different between the 2 tests. Categorical variables were analyzed with the Fisher exact test. Values are reported as means and SDs unless otherwise noted. Probability values of <0.05 were considered statistically significant, and all tests were 2 sided. Statistical analyses were performed with SPSS for Windows (version 12.0, SPSS). Sample size calculations were performed with nQuery Advisor (version 5.0). Prism for Windows 4 (version 4.02, GraphPad Software Inc.) was used for graphical presentation.
Previous SectionNext Section
Results
Baseline Information
We enrolled 15 patients with POTS and 14 controls. The baseline characteristics are enumerated in Table 1. There was no significant difference in baseline characteristics between patients with POTS and control subjects for the overall group or when just the female subjects were considered.
View this table:
In this window
In a new window
TABLE 1. Baseline Information
Supine and Upright Posture Study
As seen in Figure 1A, patients with POTS had a higher heart rate than control subjects both when supine (77±12 versus 64±12 bpm, P=0.008) and when standing upright for up to 30 minutes (128±18 versus 80±11 bpm, P<0.001). As would be expected on the basis of the diagnostic criteria for POTS, patients with POTS had a significantly greater increase in heart rate (51±18 bpm) on assuming the upright position than did the control subjects (16±10 bpm, P<0.001). The supine systolic blood pressure was similar between the 2 groups (POTS versus control, 111±14 versus 114±13 mm Hg; P=0.525; Figure 1B). Both groups experienced a small increase in systolic blood pressure that was not statistically significant on standing, with no difference between groups (POTS versus control, 123±20 versus 115±14 mm Hg; P=0.244). Neither the diastolic blood pressures while supine (POTS 67±8 mm Hg; control 70±9 mm Hg; P=0.321) or standing (POTS 79±14 mm Hg; control 77±10 mm Hg; P=0.636) nor the mean blood pressures while supine (POTS 81±9 mm Hg; control 85±10 mm Hg;P=0.367) or standing (POTS 94±15 mm Hg; control 90±10 mm Hg; P=0.417) were different between the 2 groups.

View larger version:
In this page
In a new window
Download as PowerPoint Slide
Figure 1. Hemodynamic parameters and catecholamines: supine and upright. Supine and upright values for heart rate (A), systolic blood pressure (BP; B), venous plasma norepinephrine (C), and venous plasma epinephrine (D) for patients with POTS and control subjects. Error bars represent SE. Probability values are from between-group comparison with Studentt tests.
Plasma catecholamines were drawn with subjects in both the supine and upright positions. The supine plasma norepinephrine (Figure 1C) values appeared to be slightly higher in patients with POTS (1.37±0.62 nmol/L) than in the control subjects (0.98±0.45 nmol/L), but this difference was not statistically significant (P=0.073). In response to standing, plasma norepinephrine increased significantly more in patients with POTS (>3-fold increase in norepinephrine) than in the control subjects (>2-fold increase in norepinephrine; P=0.002). Patients with POTS had a significantly higher upright plasma norepinephrine than did the control subjects (4.76±2.37 versus 2.44±0.89 nmol/L, P=0.002). In contrast to norepinephrine values, plasma epinephrine values were not different between the 2 groups in either the supine or the upright positions (Figure 1D), nor was the increase in epinephrine from supine to an upright position significantly different between the groups (P=0.350).
Renin and Aldosterone
After 3 days of the study’s fixed dietary salt intake, there was no difference in plasma renin activity (Figure 2A) between the patients with POTS and the control groups in the supine position (0.79±0.58 versus 0.79±0.74 ng · mL−1 · h−1, P=0.996). In contrast to the standing plasma norepinephrine values, there was no difference in standing plasma renin activity between patients with POTS and control subjects (2.03±1.26 versus 2.08±2.05 ng · mL−1 · h−1; P=0.944). The supine serum aldosterone (Figure 2B) was significantly lower in patients with POTS (190±140 pmol/L) than in control subjects (380±230 pmol/L; P=0.017). Serum aldosterone increased in both groups on standing. The upright serum aldosterone was significantly lower in patients with POTS (480±290 pmol/L) than in control subjects (810±370 pmol/L;P=0.019). The supine aldosterone-plasma renin activity ratio, a measure of the relationship between these 2 parameters, was significantly lower in patients with POTS (0.93±0.65) than in control subjects (3.19±3.29; P=0.047; Figure 2C).

View larger version:
In this page
In a new window
Download as PowerPoint Slide
Figure 2. Renin and aldosterone. Supine and upright values for plasma renin activity (A) and serum aldosterone (B) for patients with POTS and control subjects. C, Aldosterone to renin ratio (in conventional units) in supine position, as measure of mismatch between renin activity and aldosterone response, for patients with POTS and control subjects. Ratio of aldosterone to plasma renin activity was calculated with conventional units for aldosterone (ng/dL; 1 ng/dL=27.7 pmol/L) and plasma renin activity (ng · mL−1 · h−1) and reported without units. Error bars represent SE. Probability values are from between-group comparison with Student t test.
Supine and Upright Posture Study in Females
The hemodynamic and biochemical responses of the female subjects to supine and standing posture are shown in the figure in the online-only Data Supplement. The results were qualitatively similar to those seen in the overall group analysis. In the standing position, female patients with POTS had a significantly higher heart rate (P<0.001), a significantly higher plasma norepinephrine level (P=0.025), and a significantly lower aldosterone level (P=0.038) than control subjects. There were no differences between the groups while standing for blood pressure, plasma epinephrine, or plasma renin activity. In the setting of the smaller sample size in this subgroup analysis, none of the supine hemodynamic and biochemical parameters were significantly different between groups.
Blood Volumes
As shown in Table 2, plasma volume was significantly lower in patients with POTS (2348±438 mL) than in control subjects (2823±480 mL, P=0.010), whereas ideal plasma volumes were not significantly different. Calculating the plasma volume deficit controls for individual variations in ideal plasma volumes based on size and gender. Patients with POTS had a plasma volume deficit of 334±187 mL, which represented 12.8±7.6% of their ideal plasma volume, whereas control subjects had no deficit (10±250 mL [0.8±8.8%]). Figure 3A illustrates both this highly significant difference and the variability in plasma volume deficits within the 2 groups. The plasma volume of 1 patient with POTS actually exceeded expectations. Conversely, 3 patients with POTS had a plasma volume deficit of >20%, with a plasma volume deficit as high as 27% in 1 patient with POTS.
View this table:
In this window
In a new window
TABLE 2. Blood Volumes

View larger version:
In this page
In a new window
Download as PowerPoint Slide
Figure 3. Blood volumes. Individual and mean values are presented for plasma volume (PV) deficit (A) as percentage (individual deficit=[ideal plasma volume−measured plasma volume]/ideal plasma volume) for control subjects and patients with POTS. Negative value for deficit implies excess in plasma volume. Probability values are from between-group comparison with Student t test. Similar figures are presented for red blood cell volume deficit (B) and total blood volume (TBV) deficit (C). D through F, Data when only female subjects were included.
The measured red blood cell volume was also lower by a mean of >300 mL in patients with POTS than in control subjects (Table 2). Control subjects experienced a modest deficit in red blood cell volume. In contrast, patients with POTS had a mean deficit in red blood cell volume of >350 mL, which represented a 22.7% deficit from the expected red blood cell volume (Figure 3B). The difference between the 2 groups was highly significant (P=0.003). Supine hematocrit values were not different between patients with POTS and control subjects (37.8±2.4% versus 38.0±3.0%,P=0.812), which reflects the parallel decrease in both plasma volume and red blood cell volume in patients with POTS.
The total blood volume followed the same pattern as the plasma volume and red blood cell volume components. The measured total blood volume was significantly lower in patients with POTS than in control subjects (P=0.010; Table 2). Even after we corrected for individual differences in ideal total blood volume, patients with POTS still had a significantly larger relative deficit in total blood volume (16.5±6.8% versus 5.6±7.8%, P<0.001; Figure 3C). This works out to a mean absolute total blood volume deficit of 460 mL compared with the control subjects.
Blood volumes for the female subjects are shown in Figures 3D through 3F and in Table 2. Concordant with the overall group, each of the 3 measured blood volume deficits was greater for patients with POTS than for control groups.
Plasma Volume Shifts With Upright Tilt
Plasma volume shifts with upright tilt were calculated both early during the tilt (5 minutes [ΔPV%5 minutes]) and near the end of tilt (ΔPV%End) as a percentage of the baseline plasma volume. Neither the mean ΔPV%5 minutes (−11.7±3.2% versus −10.4±3.5%,P=0.298) nor the mean ΔPV%End (−16.6±4.7% versus −15.3±4.6%,P=0.463) was different in patients with POTS compared with control subjects. As can be seen in Figure 4, there was significant heterogeneity in the maximal plasma volume shift with upright tilt in both groups.

View larger version:
In this page
In a new window
Download as PowerPoint Slide
Figure 4. Plasma volume shift with upright tilt. Individual and mean shifts in plasma volume (PV Shift), as percentage of baseline plasma volume, in response to upright tilt are presented for control subjects and patients with POTS. Negative value represents shift of plasma volume from intravascular to extravascular space. Mean differences were not statistically significant between groups. Max indicates maximum.
Previous SectionNext Section
Discussion
This study sought to assess both blood volume and the role of the renin-angiotensin-aldosterone system in the regulation of blood volume in POTS. The main findings from this prospective study were that compared with control subjects, patients with POTS (1) have a significant deficit of plasma volume, (2) have a significantly lower level of serum aldosterone, (3) have an inappropriately low level of plasma renin activity given the degree of hypovolemia that they exhibit, and (4) have a significant deficit of red blood cell volume in the setting of an elevated standing heart rate and plasma norepinephrine.
Plasma Volume
Patients with POTS had a lower basal plasma volume (Table 2) than the control subjects. Accurate plasma volume assessments can be affected by several environmental, dietary, and patient-related factors.25 These variables may in part explain the mixed results obtained by other investigators who have tried to assess plasma volume in patients with POTS.8,9,11,12 Several measures were undertaken in the present study to ensure that the plasma volume was measured accurately. Study subjects were withdrawn from all medications that might alter the plasma volume (such as fludrocortisone) for at least 5 days before the study. They consumed a controlled sodium diet for at least 3 days before assessment, because sodium intake can alter activation of the renin-angiotensin-aldosterone axis and subsequently alter plasma volume. Finally, all plasma volume assessments were performed in the same temperature-controlled room on the research unit after the subjects had been supine for at least 1 hour. Other factors that can physiologically alter plasma volume include the subject’s size and gender.21,22 To correct for individual variations in subject size, we calculated the expected plasma volume for each subject based on his or her size and gender and subtracted the measured plasma volume to arrive at the individual’s actual plasma volume deficit, as shown in Figure 3A. Patients with POTS had a mean plasma volume deficit of almost 350 mL, whereas control subjects had no such deficit. The deficit of 13% in patients with POTS constitutes a moderate to severe hypovolemia. This reduction in effective circulating volume could trigger a cascade of perturbations associated with POTS. In the supine position, this hypovolemia may cause only modest or nonsignificant changes in heart rate and plasma norepinephrine. In the upright position, in the setting of gravitational blood pooling, the additional reduced volume could decrease the cardiac output and cause a reflex increase in sympathetic nerve activity. The result would be an increase in the upright plasma norepinephrine levels and an increase in standing heart rate, as seen in patients with POTS (Table 1).
Renin-Aldosterone Paradox
The renin-angiotensin-aldosterone system plays an important role in the regulation of plasma volume.13 Hypovolemia, acting via reduced renal blood flow and possible cardiorenal mechanisms,26,27 would be expected to increase plasma renin activity, which should augment levels of angiotensin II and subsequently lead to increased levels of aldosterone. Angiotensin II promotes renal sodium retention both directly, through receptors in the renal proximal tubule,14 and indirectly, by stimulating the secretion of the mineralocorticoid aldosterone.15Through this augmented sodium retention, the renin-angiotensin-aldosterone axis should restore extracellular fluid volume (Figure 5A).

View larger version:
In this page
In a new window
Download as PowerPoint Slide
Figure 5. Aldosterone paradox in POTS. A, Under normal circumstances, low plasma volume is sensed in kidney (and in heart and aorta) and stimulates increase in plasma renin activity (renin), angiotensin II (A-II), and aldosterone (ALDO). Increase in plasma renin activity and aldosterone promotes salt and water retention, which leads to increase in extracellular fluid volume and plasma volume. B, In POTS, there is failure to sense and appropriately respond to low plasma volume. There is no appropriate increase in plasma renin activity, angiotensin-II, and aldosterone given the hypovolemia. Because plasma renin activity and aldosterone are not increased, salt and water retention is not increased, and plasma volume is not increased.
Plasma renin activity was similar in patients with POTS and control subjects, whereas supine and upright levels of aldosterone were significantly lower in patients with POTS (Figure 2). Given their degree of hypovolemia, however, one would expect both plasma renin activity and aldosterone levels to be significantly higher in the POTS group than in controls. Both the plasma renin activity and, to a greater extent, aldosterone levels were inappropriately low given the hypovolemic status of the patients with POTS. We have termed this dysregulation of plasma renin activity and aldosterone in POTS the “renin-aldosterone paradox” (Figure 5B).
Aldosterone secretion is controlled at many levels: it is stimulated by angiotensin II, potassium, and hyponatremia, and acutely by the adrenocorticotropic hormone; it is inhibited by dopamine and atrial natriuretic factor (ANF).28 Electrolyte abnormalities are not likely to explain the low aldosterone as the sodium and potassium levels were similar in the POTS group and controls (Table 1). Although we cannot exclude the possibility that there are abnormalities in ANF or increases in adrenal dopamine concentrations that could contribute to the low aldosterone state, the most likely explanation for the renin-aldosterone paradox is an inappropriately low level of angiotensin II.
The cause of the inappropriately low levels of plasma renin activity and aldosterone in POTS is not clear. Low-flow states across the juxtaglomerular apparatus (as is seen in renal artery stenosis) are known to increase plasma renin activity. It is possible that the opposite effect somehow occurs in POTS, presumably by impaired vascular function. Other possibilities include problems with the sensor mechanisms at the level of the macula densa, or in the transmission of this signal to the juxtaglomerular apparatus, or in the response of the juxtaglomerular apparatus.13 It is also possible that patients with POTS have a low blood vessel “capacitance” that in turn limits the blood volume. Such a phenomenon has been proposed to exist in patients with pheochromocytoma, who have low blood volume.29,30 Plasma volume and total blood volume increase in response to treatment with an α-adrenergic antagonist.31
Red Blood Cell Volume
In addition to deficits in plasma volume patients with POTS also had a significant reduction in red blood cell volume (Figure 3B andTable 2). This finding would not have been apparent on cursory assessment. Hematocrit levels were not different between the 2 groups (Table 1). Because there was a parallel reduction in the 2 largest components of blood volume (plasma volume and red blood cell volume), the percentage of the blood column that was due to the red blood cells appeared to be normal. Thus, we required a formal radioisotope dilution assessment of blood volumes to document the red blood cell volume deficit.
This red blood cell volume deficit has been observed previously in patients with POTS.11,12 The pathogenesis of this deficit, however, is not known. The renal hormone erythropoietin is the primary agonist for red blood cell production in the bone marrow.32–34 It is possible that a deficit in erythropoietin production might play a pathophysiological role in POTS, although this is not yet clear.
There are several pieces of evidence that point to an important role for angiotensin II and the renin-angiotensin-aldosterone axis in the regulation of erythropoietin production. First, in healthy subjects, infusions of angiotensin II caused serum erythropoietin levels to increase significantly, but this stimulation of erythropoietin was blocked when the subjects were premedicated with losartan, an angiotensin receptor blocker.35,36 Second, plasma renin activity is higher among hemodialysis patients who do not require exogenous erythropoietin to maintain a hematocrit of 30% (which suggests adequate endogenous erythropoietin) than in those patients who require exogenous erythropoietin.37 Third, plasma ultrafiltration induced a doubling of plasma renin activity, which was accompanied by a 69% rise in serum erythropoietin over 4 hours.37 This increase in erythropoietin was abolished with the use of an ACE inhibitor. Finally, some patients develop a persistently elevated hematocrit after renal transplant.38Inactivation of the renin-angiotensin-aldosterone system by an ACE inhibitor or an angiotensin receptor blocker can correct this polycythemia,39,40 and conversely, withdrawal of the ACE inhibitor has been associated with “rebound” polycythemia.38 Taken together, these pieces of evidence suggest that the paradoxically low renin activity seen in patients with POTS could be the cause of the low red blood cell volume through a direct hormonal effect.
Another possible explanation for the low red blood cell volume seen in POTS is that it is a direct result of the low plasma volume. The kidney may function as the key organ involved in the regulation of hematocrit, because it controls both plasma volume (through salt regulation) and red blood cell volume (through erythropoietin).41 To maintain an appropriate hematocrit (the hematocrit was similar between patients with POTS and controls), the red blood cell volume may be adjusted downward through a physiologically reduced level of erythropoietin to match the deficit in plasma volume.
Erythropoietin replacement, by itself, is not likely to restore normal physiological function in patients with POTS. Hoeldtke et al12studied 8 patients with POTS and found 6 of those patients to have a low red blood cell volume. Treatment with open-label erythropoietin improved the red blood cell volume but did not increase the plasma volume. The orthostatic tachycardia was corrected in only 1 of their patients, although 3 patients subjectively reported feeling better.
Study Limitations
We have found that plasma renin activity and aldosterone are not appropriately regulated in patients with POTS. The low aldosterone-renin ratio seen in POTS suggests a mismatch between these 2 hormones. One limitation of the present study was that levels of angiotensin II, a biochemical link between renin and aldosterone, were not measured directly. Other potential regulators of aldosterone secretion (dopamine and ANF) and salt and water regulation (such as antidiuretic hormone, serum osmolality, and B-type natriuretic peptide) may also provide useful insights into the renin-aldosterone paradox seen in POTS. Future studies will include an assessment of these markers.
Previous SectionNext Section
Conclusions
In summary, we have found that patients with POTS have a reduction in plasma volume. They have inappropriately low levels of renin and low levels of aldosterone, 2 hormones that promote sodium retention and increase plasma volume and are regulated by the kidneys. These patients also have a significantly low volume of red blood cells. Red blood cell production is primarily stimulated by erythropoietin, a hormone that is released by the kidney. Taken together, these findings suggest that abnormalities in the kidney might be critical in the pathophysiology of POTS.
Previous SectionNext Section
Acknowledgments
This study was supported in part by National Institutes of Health grants 2P01 HL56693 and M01 RR00095 (General Clinical Research Center) from the National Institutes of Health. Dr Raj is a Vanderbilt Clinical Research Scholar, supported by a K12 grant from the National Institutes of Health. The DAXOR Corporation (New York, NY) kindly donated the equipment and supplies needed for the blood volume assessment.
Patients referred to the Vanderbilt University Autonomic Dysfunction Center with POTS between October 2002 and November 2003 were candidates for inclusion in this study. Patients met the current criteria for POTS.16 Briefly, patients developed symptoms of orthostatic intolerance accompanied by a heart rate rise ≥30 bpm (or a rate that exceeds 120 bpm) that occurred within the first 10 minutes of standing or head-up tilt, without any evidence of orthostatic hypotension (a fall in blood pressure of >20/10 mm Hg). Patients had at least a 6-month history of symptoms, in the absence of another chronic debilitating disorder or prolonged bed rest, and were at least 18 years of age. Healthy control subjects (who did not meet criteria for POTS, and were at least 18 years of age) underwent all of the same protocol elements. Patients and controls were free of medications that could impair autonomic tone17 and were not taking fludrocortisone for at least 5 days before testing. The Vanderbilt University Investigational Review Board approved this study and written informed consent was obtained from each subject before the study began.
Protocol
Study investigations were performed at the Elliot V. Newman Clinical Research Center at Vanderbilt University. For 3 days before testing, subjects consumed a diet that contained 150 mEq of sodium per day and 70 mEq of potassium per day. The diet was free of caffeine-containing beverages.
Supine and Upright Posture Study
Heart rate, blood pressure, aldosterone, plasma renin activity, and plasma norepinephrine and epinephrine were assessed after overnight rest with subjects in the supine position and again after subjects had been standing for up to 30 minutes (as tolerated). The standing test was performed to assess the hemodynamic and biochemical responses to increased central hypovolemia (accentuated by the gravitational stress). For catecholamine measurements, blood was collected in plastic syringes and immediately transferred to chilled vacuum tubes with EGTA and reduced glutathione (Amersham International PLC) and immediately put on ice. The plasma was separated by refrigerated centrifugation at −4°C and stored at −70°C until the assay. Concentrations of norepinephrine and epinephrine were measured by batch alumina extraction, followed by high-performance liquid chromatography for separation with electrochemical detection and quantification.8 Plasma renin enzymatic activity was assayed by conversion of angiotensinogen to angiotensin I by a radioimmunoassay technique (antibodies from IgG Corporation)18and reported in nanograms of angiotensin I per milliliter per hour. Blood for aldosterone was collected in chilled vacuum tubes without preservative, and the serum was extracted and sent to the laboratory on ice. Serum aldosterone was measured by radioimmunoassay (DPC Coat-a-Count, Diagnostic Products Corp). The aldosterone-to-plasma renin activity ratio was calculated with the conventional units for aldosterone (ng/dL; 1 ng/dL=27.7 pmol/L) and plasma renin activity (ng · mL−1 · h−1) and reported without units.
Blood Volume Assessment
Plasma volume was determined by the indicator dye-dilution technique. In the morning after an overnight fast, patients were placed in a supine position for a minimum of 60 minutes before collection of the baseline sample. A 20-gauge intravenous catheter was placed in an antecubital vein, and blood samples could be obtained without stasis. A baseline venous sample of 5 mL was collected before injection of the tracer. With a prefilled 1-mL syringe, up to 25 μCi of 131I-labeled human serum albumin (Volumex, Iso-Tex Diagnostics Inc) was injected into the antecubital vein and flushed with 30 mL of normal saline. Starting at 12 minutes after injection, 5 mL of venous blood was collected at 6-minute intervals until 30 minutes after injection (5 samples, including baseline sample). Hematocrit was measured in duplicate from each sample after 10-minute centrifugation at 11 500 rpm on an International Equipment Co microcapillary centrifuge and read on an International Equipment Co microcapillary tube reader. Plasma radioactivity was measured in duplicate and averaged (for each sample and a reference standard) with an automated counter (BVA-100 Blood Volume Analyzer, DAXOR Corporation). A least-squares regression of the volume of distribution at each time point was automatically performed to determine the volume of distribution at the time of injection. Plasma volume was determined as the volume of distribution of albumin.
Total blood volume was calculated from measured plasma volume and microcapillary venous antecubital hematocrit corrected for the plasma-packing ratio (0.99),19 the ratio of mean body hematocrit to peripheral (measured) hematocrit (0.91),20 and the effects of heparin within the sampling syringe (0.97).21
Red blood cell volume was calculated as the difference between total blood volume and plasma volume. This DAXOR method of red blood cell volume assessment was recently found to correlate well with the traditional 51Cr red blood cell-labeling method (Pearson correlation R=0.96), with a mean difference between the techniques of 0.9% (personal communication with Dr. Howard Dworkin, William Beaumont Hospital, Royal Oak, Mich).
Ideal plasma and total blood volume was determined on the basis of the height, weight, and gender of the individual subject.22Individual “deficits” in plasma volume, red blood cell volume, and total blood volume were calculated as the ideal minus measured volume (in milliliters), or this difference divided by the ideal volume (percentage).
Plasma Volume Shift With Upright Tilt
All studies occurred between 10 AM and noon in a quiet, dimly lit room at a comfortable ambient temperature (21°C to 24°C). An antecubital venous catheter was inserted (if not already in situ and functioning) for blood sampling at least 15 minutes before the beginning of the test, with the patient supine. Subjects were tilted head-up to 60° for 30 minutes or until the subject experienced presyncope that required test termination.
Blood was drawn at baseline and then at 5, 10, 15, 20, and 30 minutes of tilt for measurement of hematocrit in quadruplicate (see Blood Volume Assessment section for details of hematocrit assessment). Relative changes in hematocrit from baseline were used to calculate the change in plasma volume with upright tilt. The percentage change in plasma volume (ΔPV%)=100×[(HctBaseline−HctTime)/HctTime]× [1/(1−HctBaseline)], with the absolute change in plasma volume (ΔPV)=ΔPV%×measured plasma volume (where HctBaseline is hematocrit before tilt, and HctTime is hematocrit at a given time after tilt).23 ΔPVEnd and ΔPV%End were defined as the ΔPV at the time of the last measured hematocrit before tilt termination. ΔPVEnd was used in place of the individual late time points to minimize the confounding effect of late data dropout due to premature tilt termination. A negative value reflects a shift in plasma volume out of the vascular space.
Gender Analysis
POTS is a disorder that affects women more often than men. In addition to an overall analysis that included all subjects, a separate analysis was performed that included only female subjects. This was to ensure that the results were not skewed by the small number of men.
Statistical Analysis and Sample Size Calculations
Our primary endpoint was the plasma volume deficit (measured plasma volume minus ideal plasma volume). The null hypothesis was that the plasma volume deficit would not be statistically different between patients with POTS and control subjects. We calculated the size of our required sample after determining that a 7.5% deficit in plasma volume in patients with POTS would be clinically significant. We did not expect the control subjects to have a plasma volume deficit. Assuming a pooled SD of 5% (giving an effect size of 1.5), a sample size of 13 subjects in each group would give 95% power to detect a statistically significant difference with a Student t test with a 2-sided significance level of 0.05.24Differences between groups were analyzed with the Student t test. The Mann-Whitney U test was also used to confirm the results obtained from the Student t test, and the significance of the reported parameters was not different between the 2 tests. Categorical variables were analyzed with the Fisher exact test. Values are reported as means and SDs unless otherwise noted. Probability values of <0.05 were considered statistically significant, and all tests were 2 sided. Statistical analyses were performed with SPSS for Windows (version 12.0, SPSS). Sample size calculations were performed with nQuery Advisor (version 5.0). Prism for Windows 4 (version 4.02, GraphPad Software Inc.) was used for graphical presentation.
Previous SectionNext Section
Results
Baseline Information
We enrolled 15 patients with POTS and 14 controls. The baseline characteristics are enumerated in Table 1. There was no significant difference in baseline characteristics between patients with POTS and control subjects for the overall group or when just the female subjects were considered.
View this table:
In this window
In a new window
TABLE 1. Baseline Information
Supine and Upright Posture Study
As seen in Figure 1A, patients with POTS had a higher heart rate than control subjects both when supine (77±12 versus 64±12 bpm, P=0.008) and when standing upright for up to 30 minutes (128±18 versus 80±11 bpm, P<0.001). As would be expected on the basis of the diagnostic criteria for POTS, patients with POTS had a significantly greater increase in heart rate (51±18 bpm) on assuming the upright position than did the control subjects (16±10 bpm, P<0.001). The supine systolic blood pressure was similar between the 2 groups (POTS versus control, 111±14 versus 114±13 mm Hg; P=0.525; Figure 1B). Both groups experienced a small increase in systolic blood pressure that was not statistically significant on standing, with no difference between groups (POTS versus control, 123±20 versus 115±14 mm Hg; P=0.244). Neither the diastolic blood pressures while supine (POTS 67±8 mm Hg; control 70±9 mm Hg; P=0.321) or standing (POTS 79±14 mm Hg; control 77±10 mm Hg; P=0.636) nor the mean blood pressures while supine (POTS 81±9 mm Hg; control 85±10 mm Hg;P=0.367) or standing (POTS 94±15 mm Hg; control 90±10 mm Hg; P=0.417) were different between the 2 groups.

View larger version:
In this page
In a new window
Download as PowerPoint Slide
Figure 1. Hemodynamic parameters and catecholamines: supine and upright. Supine and upright values for heart rate (A), systolic blood pressure (BP; B), venous plasma norepinephrine (C), and venous plasma epinephrine (D) for patients with POTS and control subjects. Error bars represent SE. Probability values are from between-group comparison with Studentt tests.
Plasma catecholamines were drawn with subjects in both the supine and upright positions. The supine plasma norepinephrine (Figure 1C) values appeared to be slightly higher in patients with POTS (1.37±0.62 nmol/L) than in the control subjects (0.98±0.45 nmol/L), but this difference was not statistically significant (P=0.073). In response to standing, plasma norepinephrine increased significantly more in patients with POTS (>3-fold increase in norepinephrine) than in the control subjects (>2-fold increase in norepinephrine; P=0.002). Patients with POTS had a significantly higher upright plasma norepinephrine than did the control subjects (4.76±2.37 versus 2.44±0.89 nmol/L, P=0.002). In contrast to norepinephrine values, plasma epinephrine values were not different between the 2 groups in either the supine or the upright positions (Figure 1D), nor was the increase in epinephrine from supine to an upright position significantly different between the groups (P=0.350).
Renin and Aldosterone
After 3 days of the study’s fixed dietary salt intake, there was no difference in plasma renin activity (Figure 2A) between the patients with POTS and the control groups in the supine position (0.79±0.58 versus 0.79±0.74 ng · mL−1 · h−1, P=0.996). In contrast to the standing plasma norepinephrine values, there was no difference in standing plasma renin activity between patients with POTS and control subjects (2.03±1.26 versus 2.08±2.05 ng · mL−1 · h−1; P=0.944). The supine serum aldosterone (Figure 2B) was significantly lower in patients with POTS (190±140 pmol/L) than in control subjects (380±230 pmol/L; P=0.017). Serum aldosterone increased in both groups on standing. The upright serum aldosterone was significantly lower in patients with POTS (480±290 pmol/L) than in control subjects (810±370 pmol/L;P=0.019). The supine aldosterone-plasma renin activity ratio, a measure of the relationship between these 2 parameters, was significantly lower in patients with POTS (0.93±0.65) than in control subjects (3.19±3.29; P=0.047; Figure 2C).

View larger version:
In this page
In a new window
Download as PowerPoint Slide
Figure 2. Renin and aldosterone. Supine and upright values for plasma renin activity (A) and serum aldosterone (B) for patients with POTS and control subjects. C, Aldosterone to renin ratio (in conventional units) in supine position, as measure of mismatch between renin activity and aldosterone response, for patients with POTS and control subjects. Ratio of aldosterone to plasma renin activity was calculated with conventional units for aldosterone (ng/dL; 1 ng/dL=27.7 pmol/L) and plasma renin activity (ng · mL−1 · h−1) and reported without units. Error bars represent SE. Probability values are from between-group comparison with Student t test.
Supine and Upright Posture Study in Females
The hemodynamic and biochemical responses of the female subjects to supine and standing posture are shown in the figure in the online-only Data Supplement. The results were qualitatively similar to those seen in the overall group analysis. In the standing position, female patients with POTS had a significantly higher heart rate (P<0.001), a significantly higher plasma norepinephrine level (P=0.025), and a significantly lower aldosterone level (P=0.038) than control subjects. There were no differences between the groups while standing for blood pressure, plasma epinephrine, or plasma renin activity. In the setting of the smaller sample size in this subgroup analysis, none of the supine hemodynamic and biochemical parameters were significantly different between groups.
Blood Volumes
As shown in Table 2, plasma volume was significantly lower in patients with POTS (2348±438 mL) than in control subjects (2823±480 mL, P=0.010), whereas ideal plasma volumes were not significantly different. Calculating the plasma volume deficit controls for individual variations in ideal plasma volumes based on size and gender. Patients with POTS had a plasma volume deficit of 334±187 mL, which represented 12.8±7.6% of their ideal plasma volume, whereas control subjects had no deficit (10±250 mL [0.8±8.8%]). Figure 3A illustrates both this highly significant difference and the variability in plasma volume deficits within the 2 groups. The plasma volume of 1 patient with POTS actually exceeded expectations. Conversely, 3 patients with POTS had a plasma volume deficit of >20%, with a plasma volume deficit as high as 27% in 1 patient with POTS.
View this table:
In this window
In a new window
TABLE 2. Blood Volumes

View larger version:
In this page
In a new window
Download as PowerPoint Slide
Figure 3. Blood volumes. Individual and mean values are presented for plasma volume (PV) deficit (A) as percentage (individual deficit=[ideal plasma volume−measured plasma volume]/ideal plasma volume) for control subjects and patients with POTS. Negative value for deficit implies excess in plasma volume. Probability values are from between-group comparison with Student t test. Similar figures are presented for red blood cell volume deficit (B) and total blood volume (TBV) deficit (C). D through F, Data when only female subjects were included.
The measured red blood cell volume was also lower by a mean of >300 mL in patients with POTS than in control subjects (Table 2). Control subjects experienced a modest deficit in red blood cell volume. In contrast, patients with POTS had a mean deficit in red blood cell volume of >350 mL, which represented a 22.7% deficit from the expected red blood cell volume (Figure 3B). The difference between the 2 groups was highly significant (P=0.003). Supine hematocrit values were not different between patients with POTS and control subjects (37.8±2.4% versus 38.0±3.0%,P=0.812), which reflects the parallel decrease in both plasma volume and red blood cell volume in patients with POTS.
The total blood volume followed the same pattern as the plasma volume and red blood cell volume components. The measured total blood volume was significantly lower in patients with POTS than in control subjects (P=0.010; Table 2). Even after we corrected for individual differences in ideal total blood volume, patients with POTS still had a significantly larger relative deficit in total blood volume (16.5±6.8% versus 5.6±7.8%, P<0.001; Figure 3C). This works out to a mean absolute total blood volume deficit of 460 mL compared with the control subjects.
Blood volumes for the female subjects are shown in Figures 3D through 3F and in Table 2. Concordant with the overall group, each of the 3 measured blood volume deficits was greater for patients with POTS than for control groups.
Plasma Volume Shifts With Upright Tilt
Plasma volume shifts with upright tilt were calculated both early during the tilt (5 minutes [ΔPV%5 minutes]) and near the end of tilt (ΔPV%End) as a percentage of the baseline plasma volume. Neither the mean ΔPV%5 minutes (−11.7±3.2% versus −10.4±3.5%,P=0.298) nor the mean ΔPV%End (−16.6±4.7% versus −15.3±4.6%,P=0.463) was different in patients with POTS compared with control subjects. As can be seen in Figure 4, there was significant heterogeneity in the maximal plasma volume shift with upright tilt in both groups.

View larger version:
In this page
In a new window
Download as PowerPoint Slide
Figure 4. Plasma volume shift with upright tilt. Individual and mean shifts in plasma volume (PV Shift), as percentage of baseline plasma volume, in response to upright tilt are presented for control subjects and patients with POTS. Negative value represents shift of plasma volume from intravascular to extravascular space. Mean differences were not statistically significant between groups. Max indicates maximum.
Previous SectionNext Section
Discussion
This study sought to assess both blood volume and the role of the renin-angiotensin-aldosterone system in the regulation of blood volume in POTS. The main findings from this prospective study were that compared with control subjects, patients with POTS (1) have a significant deficit of plasma volume, (2) have a significantly lower level of serum aldosterone, (3) have an inappropriately low level of plasma renin activity given the degree of hypovolemia that they exhibit, and (4) have a significant deficit of red blood cell volume in the setting of an elevated standing heart rate and plasma norepinephrine.
Plasma Volume
Patients with POTS had a lower basal plasma volume (Table 2) than the control subjects. Accurate plasma volume assessments can be affected by several environmental, dietary, and patient-related factors.25 These variables may in part explain the mixed results obtained by other investigators who have tried to assess plasma volume in patients with POTS.8,9,11,12 Several measures were undertaken in the present study to ensure that the plasma volume was measured accurately. Study subjects were withdrawn from all medications that might alter the plasma volume (such as fludrocortisone) for at least 5 days before the study. They consumed a controlled sodium diet for at least 3 days before assessment, because sodium intake can alter activation of the renin-angiotensin-aldosterone axis and subsequently alter plasma volume. Finally, all plasma volume assessments were performed in the same temperature-controlled room on the research unit after the subjects had been supine for at least 1 hour. Other factors that can physiologically alter plasma volume include the subject’s size and gender.21,22 To correct for individual variations in subject size, we calculated the expected plasma volume for each subject based on his or her size and gender and subtracted the measured plasma volume to arrive at the individual’s actual plasma volume deficit, as shown in Figure 3A. Patients with POTS had a mean plasma volume deficit of almost 350 mL, whereas control subjects had no such deficit. The deficit of 13% in patients with POTS constitutes a moderate to severe hypovolemia. This reduction in effective circulating volume could trigger a cascade of perturbations associated with POTS. In the supine position, this hypovolemia may cause only modest or nonsignificant changes in heart rate and plasma norepinephrine. In the upright position, in the setting of gravitational blood pooling, the additional reduced volume could decrease the cardiac output and cause a reflex increase in sympathetic nerve activity. The result would be an increase in the upright plasma norepinephrine levels and an increase in standing heart rate, as seen in patients with POTS (Table 1).
Renin-Aldosterone Paradox
The renin-angiotensin-aldosterone system plays an important role in the regulation of plasma volume.13 Hypovolemia, acting via reduced renal blood flow and possible cardiorenal mechanisms,26,27 would be expected to increase plasma renin activity, which should augment levels of angiotensin II and subsequently lead to increased levels of aldosterone. Angiotensin II promotes renal sodium retention both directly, through receptors in the renal proximal tubule,14 and indirectly, by stimulating the secretion of the mineralocorticoid aldosterone.15Through this augmented sodium retention, the renin-angiotensin-aldosterone axis should restore extracellular fluid volume (Figure 5A).

View larger version:
In this page
In a new window
Download as PowerPoint Slide
Figure 5. Aldosterone paradox in POTS. A, Under normal circumstances, low plasma volume is sensed in kidney (and in heart and aorta) and stimulates increase in plasma renin activity (renin), angiotensin II (A-II), and aldosterone (ALDO). Increase in plasma renin activity and aldosterone promotes salt and water retention, which leads to increase in extracellular fluid volume and plasma volume. B, In POTS, there is failure to sense and appropriately respond to low plasma volume. There is no appropriate increase in plasma renin activity, angiotensin-II, and aldosterone given the hypovolemia. Because plasma renin activity and aldosterone are not increased, salt and water retention is not increased, and plasma volume is not increased.
Plasma renin activity was similar in patients with POTS and control subjects, whereas supine and upright levels of aldosterone were significantly lower in patients with POTS (Figure 2). Given their degree of hypovolemia, however, one would expect both plasma renin activity and aldosterone levels to be significantly higher in the POTS group than in controls. Both the plasma renin activity and, to a greater extent, aldosterone levels were inappropriately low given the hypovolemic status of the patients with POTS. We have termed this dysregulation of plasma renin activity and aldosterone in POTS the “renin-aldosterone paradox” (Figure 5B).
Aldosterone secretion is controlled at many levels: it is stimulated by angiotensin II, potassium, and hyponatremia, and acutely by the adrenocorticotropic hormone; it is inhibited by dopamine and atrial natriuretic factor (ANF).28 Electrolyte abnormalities are not likely to explain the low aldosterone as the sodium and potassium levels were similar in the POTS group and controls (Table 1). Although we cannot exclude the possibility that there are abnormalities in ANF or increases in adrenal dopamine concentrations that could contribute to the low aldosterone state, the most likely explanation for the renin-aldosterone paradox is an inappropriately low level of angiotensin II.
The cause of the inappropriately low levels of plasma renin activity and aldosterone in POTS is not clear. Low-flow states across the juxtaglomerular apparatus (as is seen in renal artery stenosis) are known to increase plasma renin activity. It is possible that the opposite effect somehow occurs in POTS, presumably by impaired vascular function. Other possibilities include problems with the sensor mechanisms at the level of the macula densa, or in the transmission of this signal to the juxtaglomerular apparatus, or in the response of the juxtaglomerular apparatus.13 It is also possible that patients with POTS have a low blood vessel “capacitance” that in turn limits the blood volume. Such a phenomenon has been proposed to exist in patients with pheochromocytoma, who have low blood volume.29,30 Plasma volume and total blood volume increase in response to treatment with an α-adrenergic antagonist.31
Red Blood Cell Volume
In addition to deficits in plasma volume patients with POTS also had a significant reduction in red blood cell volume (Figure 3B andTable 2). This finding would not have been apparent on cursory assessment. Hematocrit levels were not different between the 2 groups (Table 1). Because there was a parallel reduction in the 2 largest components of blood volume (plasma volume and red blood cell volume), the percentage of the blood column that was due to the red blood cells appeared to be normal. Thus, we required a formal radioisotope dilution assessment of blood volumes to document the red blood cell volume deficit.
This red blood cell volume deficit has been observed previously in patients with POTS.11,12 The pathogenesis of this deficit, however, is not known. The renal hormone erythropoietin is the primary agonist for red blood cell production in the bone marrow.32–34 It is possible that a deficit in erythropoietin production might play a pathophysiological role in POTS, although this is not yet clear.
There are several pieces of evidence that point to an important role for angiotensin II and the renin-angiotensin-aldosterone axis in the regulation of erythropoietin production. First, in healthy subjects, infusions of angiotensin II caused serum erythropoietin levels to increase significantly, but this stimulation of erythropoietin was blocked when the subjects were premedicated with losartan, an angiotensin receptor blocker.35,36 Second, plasma renin activity is higher among hemodialysis patients who do not require exogenous erythropoietin to maintain a hematocrit of 30% (which suggests adequate endogenous erythropoietin) than in those patients who require exogenous erythropoietin.37 Third, plasma ultrafiltration induced a doubling of plasma renin activity, which was accompanied by a 69% rise in serum erythropoietin over 4 hours.37 This increase in erythropoietin was abolished with the use of an ACE inhibitor. Finally, some patients develop a persistently elevated hematocrit after renal transplant.38Inactivation of the renin-angiotensin-aldosterone system by an ACE inhibitor or an angiotensin receptor blocker can correct this polycythemia,39,40 and conversely, withdrawal of the ACE inhibitor has been associated with “rebound” polycythemia.38 Taken together, these pieces of evidence suggest that the paradoxically low renin activity seen in patients with POTS could be the cause of the low red blood cell volume through a direct hormonal effect.
Another possible explanation for the low red blood cell volume seen in POTS is that it is a direct result of the low plasma volume. The kidney may function as the key organ involved in the regulation of hematocrit, because it controls both plasma volume (through salt regulation) and red blood cell volume (through erythropoietin).41 To maintain an appropriate hematocrit (the hematocrit was similar between patients with POTS and controls), the red blood cell volume may be adjusted downward through a physiologically reduced level of erythropoietin to match the deficit in plasma volume.
Erythropoietin replacement, by itself, is not likely to restore normal physiological function in patients with POTS. Hoeldtke et al12studied 8 patients with POTS and found 6 of those patients to have a low red blood cell volume. Treatment with open-label erythropoietin improved the red blood cell volume but did not increase the plasma volume. The orthostatic tachycardia was corrected in only 1 of their patients, although 3 patients subjectively reported feeling better.
Study Limitations
We have found that plasma renin activity and aldosterone are not appropriately regulated in patients with POTS. The low aldosterone-renin ratio seen in POTS suggests a mismatch between these 2 hormones. One limitation of the present study was that levels of angiotensin II, a biochemical link between renin and aldosterone, were not measured directly. Other potential regulators of aldosterone secretion (dopamine and ANF) and salt and water regulation (such as antidiuretic hormone, serum osmolality, and B-type natriuretic peptide) may also provide useful insights into the renin-aldosterone paradox seen in POTS. Future studies will include an assessment of these markers.
Previous SectionNext Section
Conclusions
In summary, we have found that patients with POTS have a reduction in plasma volume. They have inappropriately low levels of renin and low levels of aldosterone, 2 hormones that promote sodium retention and increase plasma volume and are regulated by the kidneys. These patients also have a significantly low volume of red blood cells. Red blood cell production is primarily stimulated by erythropoietin, a hormone that is released by the kidney. Taken together, these findings suggest that abnormalities in the kidney might be critical in the pathophysiology of POTS.
Previous SectionNext Section
Acknowledgments
This study was supported in part by National Institutes of Health grants 2P01 HL56693 and M01 RR00095 (General Clinical Research Center) from the National Institutes of Health. Dr Raj is a Vanderbilt Clinical Research Scholar, supported by a K12 grant from the National Institutes of Health. The DAXOR Corporation (New York, NY) kindly donated the equipment and supplies needed for the blood volume assessment.